Uncooked And Unpasteurized Foods
Validation
Quantum Food Solutions offers consultancy services for food processors making uncooked and unpasteurized foods. This category includes foods known to occasionally contain low levels of L. monocytogenes and do not have a kill step, as well as ready-to-eat refrigerated foods that have a shelf-life of ≤ 5 days. Examples of uncooked and unpasteurized foods include cold-smoked salmon, fresh-cut produce, fresh salads, fresh fruit juices.
The rationale for these products is that these products would be handled under reasonably foreseeable conditions of distribution. The stated shelf life, storage and use would be enough time for L. monocytogenes to grow to levels above 100 CFU/g before the durable life date shown as a “best before” date on the package.
These products are classified as Category 2A under Health Canada’s Listeria Policy on Ready-To-Eat foods. For these foods, processors should have food safety consultants validate and verify their processes to make sure that the levels of L. monocytogenes are consistently equal to or less than 100 CFU/g throughout the stated shelf-life.
Important Validation Concerns For Uncooked Food Products
Some of the most critical validation considerations are product characteristics, also known as growth factors (i.e. pH and Aw-value). It is important for a food processor to clearly understand what organisms are likely to survive and grow based on the characteristics of the finished product. In many cases, either the pH, the Aw-value, or a combination of the two factors may already offer some degree of food protection. However, this does not preclude the need to incorporate possible abuse conditions during consumer use as part of the initial validation study.
Given that contaminants may be introduced from a variety of sources (e.g. inadequate sanitation practices, insufficient employee hygiene, high microbial loads from other untreated raw materials such as spices, unsuitable processing environment, absence of antimicrobial agents, etc.) a product monitoring system and regular comprehensive risk assessments are necessary.
Even though Category 2A products are expected not to exceed 100 CFU/g throughout the product's shelf life, your facility should include additional control measures as part of the formulation process. These include using antimicrobials (e.g., sodium lactate as a Listeria inhibitor), and irradiating raw materials whenever possible.

Quantum Food Solutions Benefits
Uncooked and unpasteurized foods need very rigorous and scientifically-validated processes. Each control measure put in place to address the potential hazards is an essential part of your food safety system, keeping consumers safe and satisfied with your product. Quality food safety consultants will help you put this hazard-avoidance system into practice and show you areas where you can save money.
Quantum Food Solutions' validation and safety consultancy services depend on the most rugged, highest-accuracy instruments currently available. We closely monitor the different factors required to attain and maintain an adequate level of food protection. Our team is also trained to use validated predictive modelling tools such as ComBase, allowing them to effectively increase the level of food safety of your products.
Fermented / Cured Meats
Validation
Fermentation, drying, and smoking meats are probably the oldest forms of meat preservation. These two processes are mentioned together because, in practice, they are almost inseparable. These preservation methods are at least several thousands of years old, even being referenced in Homer's Odyssey!
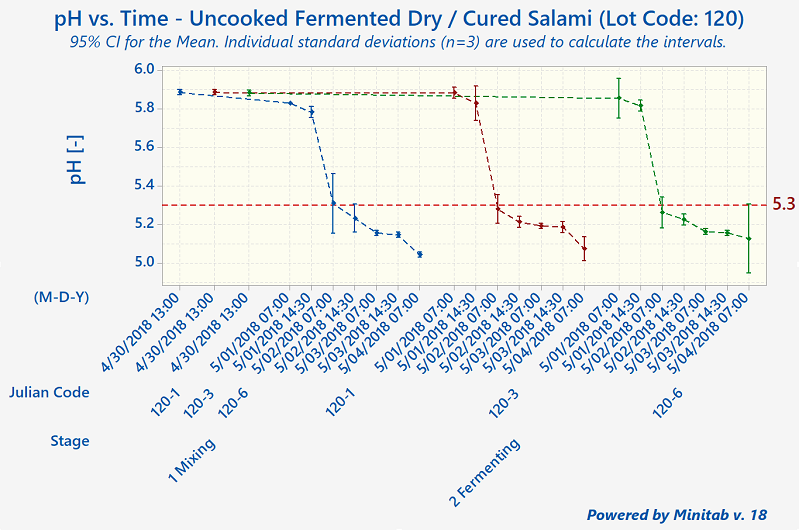
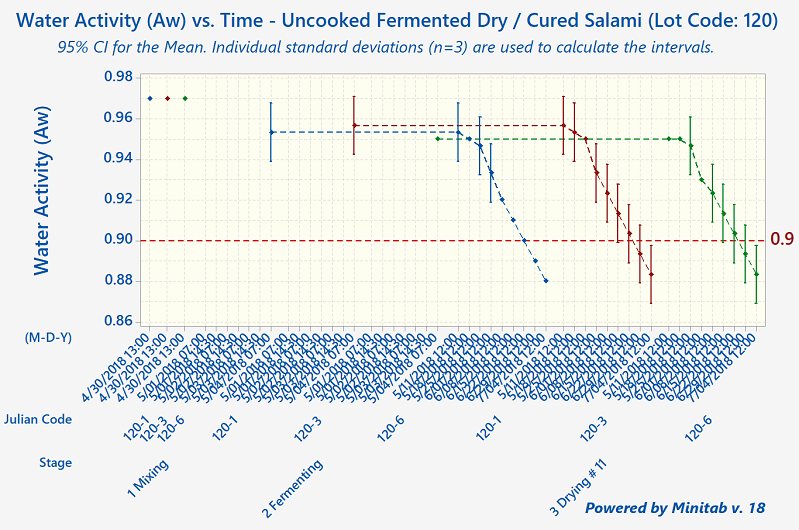
Since that time, humans have come to understand that, although these processes preserve meats well, they aren't foolproof. The modern-day validation process for uncooked fermented and dry-cured meats involves the collection of a lot of process data, including pH, Aw-value, temperature, relative humidity, and degree-hours).
Quantum Food Solutions works with companies to establish successful validation studies that meet their needs. Our food safety consultants can help your company come up with detailed descriptions of the worst-case-scenarios for the product or products under evaluation. These are based on factors such as grind size, calibre, starter culture, substrate, initial microbial load from the ingredients, cold-spots in fermentation rooms (the zone where a product has the slowest fermentation rate or pH drop), and "wettest-spots" in drying rooms (slowest drying rates).
Exporting These Products To The United States
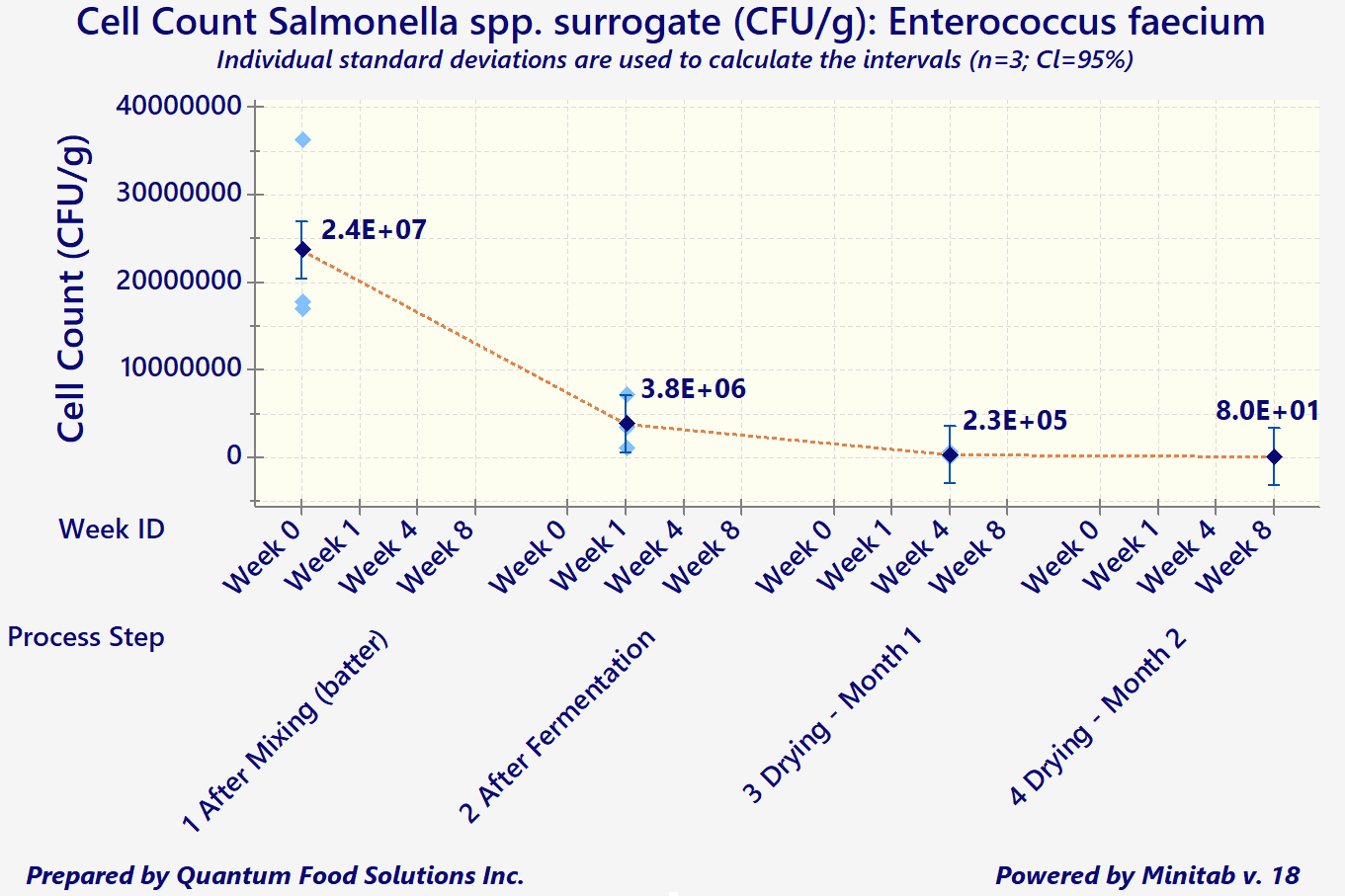
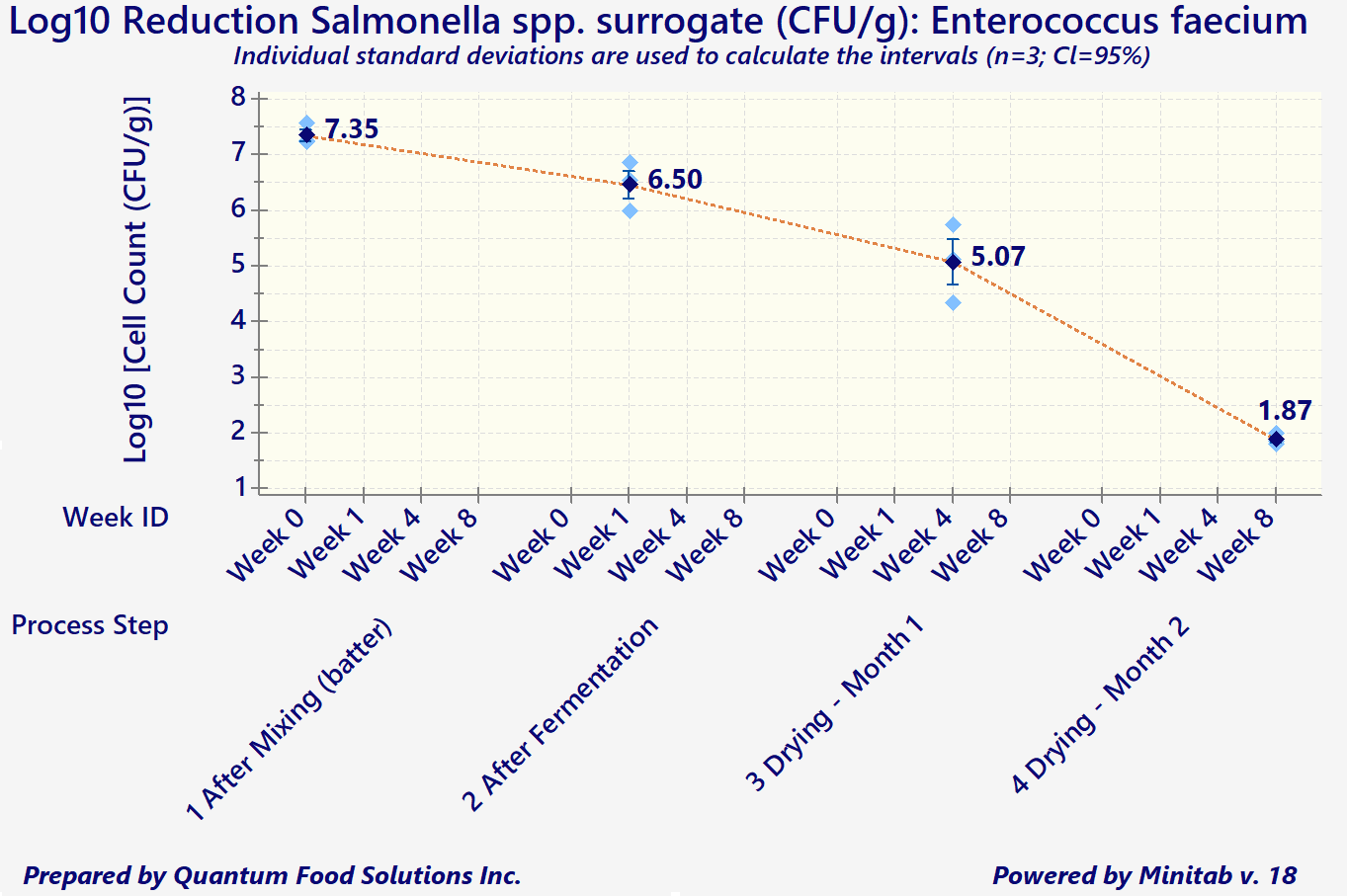
In addition to the above considerations, any processors wishing to export to the US are required to demonstrate that the existing process is capable of attaining a minimum 5-Log reduction in both Salmonella spp., and E. coli O157: H7 (for products containing beef).
"In-Plant" data should be collected through an inoculation study, per FSIS HACCP Validation Guidelines. However, since a pathogen such as Salmonella would be too dangerous to be introduced into any meat processing plant, the use of surrogate organisms is highly recommended and is also found acceptable by FSIS.

Hazards, Control Measures, And 5 -Log Reduction?
Log reduction measures the thoroughness of a decontamination process and how it reduces the concentration of a contaminant. It's defined as the common logarithm of the ratio of the levels of contamination before and after decontamination. An increment of 1 corresponds to a reduction of the contaminant concentration by a factor of 10. The ‘5-Logs’ is the difference of the exponents (base 10) between the initial and final cell counts/gram of product.
Under the current regulatory framework, the following hazards have been identified as a public concern:
Salmonella spp., & E. coli O157: H7 (products containing beef)
Under Canadian regulations, processors are allowed to use pH and Aw-value (independently or in combination). There is no actual ‘prescribed’ regulatory standard such as a 5-Log reduction. However, the 5-Log reduction does become a regulatory performance standard required for exports to the United States.
Listeria monocytogenes
Under Canadian regulations (Health Canada’s Policy on Listeria monocytogenes in RTE Foods), processors are allowed to sell these products provided that the product does not exceed a count greater than 100 CFUs/g during its usable shelf-life. This is monitored regularly through CFIA’s mandated testing of RTE products (i.e., CFIA Category 2B).
As a simple example, let's say an initial product batter starts with approximately 1,000,000 cells per gram (10^6 CFUs/g). The combination of product composition and acceptable processing parameters must decrease that number to 10 cells/gram (or 10^1 CFUs/g) by the end of the process (for meat, often it's drying).
If you need help coming up with a plan for your meat processing facility, Quantum Food Solutions uses scientific and engineering expertise to come up with a custom food safety plan.